Abstract
Therapy-resistant leukemic stem cells (LSCs) are a main cause of relapse in chronic and acute myeloid leukemia (CML and AML) patients. Stemness, a cellular state of LSCs characterized by their quiescence, pluripotency, and long-term self-renewal capacity, is thought to have major implications on therapy resistance and outcome in myeloid leukemias. However, signaling pathways that induce stemness are often not specific for LSCs and shared with normal hematopoietic stem cells (HSCs). Therefore, LSCs are difficult to be targeted therapeutically. IL-33/ST2 signaling may be a potential target to eradicate CML and AML LSCs.
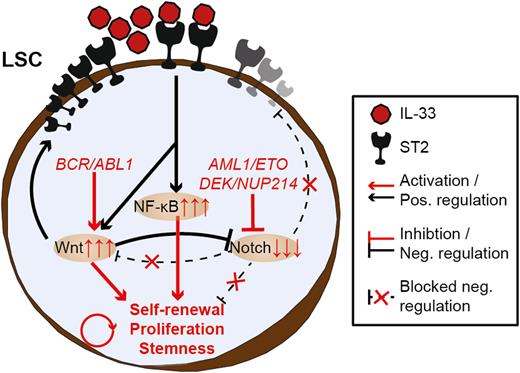
To investigate the ST2 gene (IL1RL1) expression in different hematological malignancies, we used the MILE gene expression dataset containing 2096 samples. The IL1RL1 high expressing samples (n=62) were exclusively represented by myeloid leukemias (CML and AML), and 50% of the IL1RL1 high samples harbored either the BCR/ABL1 (CML) or the AML1/ETO (AML) oncogenes. We confirmed the result from the MILE database by analyzing the ST2 protein expression on FACS-sorted CD34+ LSCs from AML and CML patient samples. Indeed, AML1/ETO and BCR/ABL1 harboring LSCs expressed ST2, while healthy hematopoietic stem cells (HSCs) and LSCs harboring other oncogenes were ST2-negative. Transfecting the ST2-negative cell line Ramos with the AML1/ETO and BCR/ABL1 oncogenes induced ST2-expression.
Next, we performed a gene set enrichment analysis (GSEA), comparing the IL1RL1 high vs. IL1RL1 low samples in the MILE dataset. Genes involved in stemness (p<0.01), Wnt- (p<0.001), and NF-ΚB (p=0.054) signaling were upregulated, while the Notch-pathway (p<0.05) was downregulated. Since the NF-ΚB is a known downstream pathway of ST2, we hypothesized that Wnt and/or Notch signaling pathways regulate ST2 expression. Therefore, we treated FACS-sorted CD34+ AML1/ETO expressing LSCs with the Wnt pathway inhibitor XAV939 or HES1 and NOTCH1 shRNAs, silencing the Notch pathway. Treatment with XAV939 led to a decrease in ST2 expression in these cells, while silencing HES1 and NOTCH1 enhanced ST2-expression.
To analyze the molecular mechanisms induced by IL-33/ST2 signaling, we performed a transcriptomic analysis using FACS-sorted CD34+ AML1/ETO LSCs upon treatment with or without IL-33. In total, 377 genes were up- and 139 genes downregulated after IL-33 stimulation. IL-33-induced genes were involved in the positive regulation of NF-ΚB (p<0.05) and canonical Wnt (p<0.001) signaling pathways, and the regulation of stem cell division and maintenance (p<0.001). To functionally test whether IL-33/ST2 signaling triggers stemness in myeloid leukemia, we performed a colony forming assay using CD34+ AML1/ETO or BCR/ABL1 expressing LSCs from leukemia patient samples. The colony numbers increased 1.5-3fold upon IL-33 treatment in three consecutive platings, indicating an increase in stem cell numbers. IL-33 had no effect on colony numbers of HSCs and LSCs harboring other oncogenes.
To test the in vivo relevance of IL-33/ST2 signaling in leukemia development, we used a congenic AML1/ETO9a (AE9a) or BCR/ABL1 (CML) mouse model. Briefly, we transduced ST2-negative HSCs originating from BL/6 or ST2-knockout (ST2-/-) mice with AE9a-GFP or BCR/ABL1-GFP and transplanted those cells into recipients. In both models, the LSCs strongly expressed ST2, but IL-33/ST2 signaling had no influence on the disease development.
Stemness supports resistance against standard therapy in both, AML and CML. Since we were able to show that IL-33/ST2 signaling promotes stemness in AML1/ETO and BCR/ABL1 LSCs, we hypothesized that IL-33 addition protects LSCs from treatment with standard therapeutics. Indeed, IL-33 rescued the reduced colony forming capacity of LSCs that were either treated with the chemotherapeutic agent cytarabine (AML1/ETO LSCs) or the tyrosine kinase inhibitor Nilotinib (BCR/ABL1 LSCs). Furthermore, ST2-deficiency led to a reduced LSC count in CML mice compared to control mice when treated with Nilotinib (p=0.047).
We showed that ST2 is expressed on AML1/ETO and BCR/ABL1 harboring LSCs and that activated IL-33/ST2 signaling induced stemness in these LSCs. Targeting IL-33/ST2 signaling in these specific subtypes of AML and CML may eliminate LSCs and overcome resistance towards chemotherapy or TKIs.
Disclosures
Döhner:Daiichi Sankyo Co, LTD: Consultancy, Honoraria; Gilead Sciences, Inc.: Consultancy, Honoraria; Astellas Pharma Inc.: Consultancy, Honoraria, Research Funding; Amgen Inc.: Consultancy, Honoraria, Research Funding; Agios Pharmaceuticals: Consultancy, Honoraria, Research Funding; AbbVie Inc.: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Syndax Pharmaceuticals Inc.: Consultancy, Honoraria; AstraZeneca: Honoraria; Berlin-Chemie: Consultancy, Honoraria; Brystol Myers Squibb: Consultancy, Honoraria, Research Funding; Janssen Pharmaceuticals: Consultancy, Honoraria; Pfizer Inc.: Research Funding; Kronos Bio, Inc.: Research Funding; Servier: Consultancy, Honoraria. Döhner:BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Research Funding; Astellas: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kronos: Research Funding. Ochsenbein:Molecular Partners AG: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.